Hypoglycemia is a very important topic for persons with diabetes to read and understand. This topic was covered in my book, The Ketogenic Diet for Type 1 Diabetes also available on Amazon in print. My other book, Conquer Type 2 Diabetes with a Ketogenic Diet, covers this topic for those with T2DM which is also available on Amazon in print. I would appreciate anyone who has read and benefited from either of these books to leave a review on Amazon. The number and ratings of the reviews are used by Amazon to order the search results when people are looking for books on diabetes.
Today, 5/15/2018, is my 58th birthday. You might be wondering how I am going celebrate this day. Cake? Champagne? No, this year I’m celebrating my CAC score results. What is a CAC score? It is short for coronary artery calcium score. I first learned about the usefulness of this noninvasive cardiac screening test from Dr. Jeffry Gerber, MD here soon after starting the ketogenic diet in 2012 when we were attending a medical meeting. Because I have had type 1 diabetes mellitus (T1DM) for 20 years and have been on the ketogenic diet for six years, I thought it was about time to take a look. As you can see below my score was zero!!!
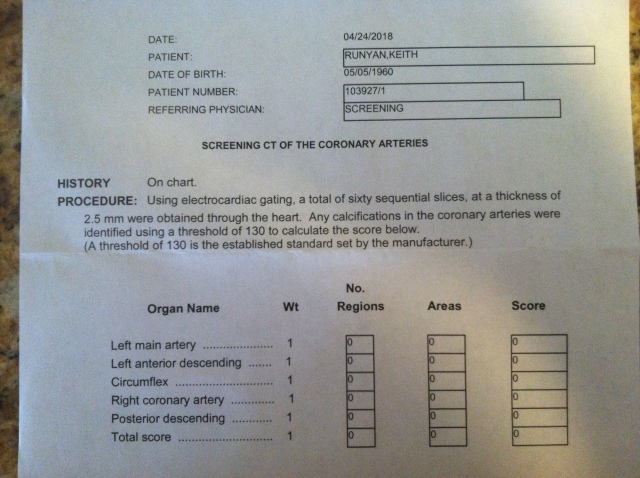
That is good news, because cardiovascular disease is the most common cause of death for those with T1DM. One less thing to think about now. I honestly believe my whole food nutrient dense ketogenic diet is cardioprotective because it is improves my glycemic control, does not cause inflammation (my hsCRP was 0.8), and results in low levels of small-dense LDL, oxidized LDL, and glycated LDL which are thought to lead to coronary artery disease. Although, I have not measured these particles, the fact that my fasting triglycerides are 65 mg/dl, HDL-C is 90 mg/dl, VLDL-C is 12 mg/dl, and remnant cholesterol is 12 mg/dl are all indicators that these particles are low and my risk for cardiac disease is relatively low. The CAC score of zero, however, is the icing on the cake which I will be enjoying on my birthday and every day. As far as what I’m eating, that will remain the same: salmon, 85% lean hamburger, one egg, steamed cabbage with some tomato sauce (no sugar added), or similar non-starchy vegetables and 7 varieties of nuts and seeds. This stays pretty constant from day to day except I substitute liverwurst or beef for salmon at breakfast time and add in home-made fermented sauerkraut 3 days a week.
Now For Today’s Topic, Hypoglycemia.
Remember the following is not medical advice, but is information that you can use with your own physician’s advice. Hypoglycemia represents a significant potential problem for those with diabetes who require insulin or any of the insulin-secreting medications (reviewed below). However for those with T1DM, hypoglycemia is a more significant problem due to the complete loss of the pancreatic β-cells and their interaction with their neighboring alpha-cells as will be explained below.
What Is Hypoglycemia? Why Does It Occur?
Hypoglycemia can be defined as an abnormally low plasma glucose concentration that exposes the individual to potential harm. Since persons with diabetes use blood glucose meters that are calibrated to give results in line with plasma glucose concentrations, I will use the term blood glucose (BG) to be equivalent to plasma glucose. A single value of BG that defines hypoglycemia is actually difficult to justify. For example in persons with poorly controlled diabetes, a BG value of 120 mg/dl (6.7 mmol/l) may result in typical symptoms of hypoglycemia whereas in persons with excellent glycemic control and especially those following a ketogenic diet may not experience symptoms until the BG falls below 50 mg/dl (2.8 mmol/l). More on this below, but first we should discuss why are those with T1DM and type 2 diabetes mellitus (T2DM) at risk for hypoglycemia?
In healthy humans BG is tightly regulated by numerous hormones and organs. Without getting too deep into the details, suffice it to say that the principle hormones that regulate BG are insulin, amylin, and glucagon and the principle organs that influence BG are the liver, muscle, and fat. Briefly, the β-cells in the pancreas secrete insulin and amylin in response to current BG and amino acid levels and are modified by other hormones including glucagon. Insulin is capable of reaching a high concentration around the neighboring alpha-cells to suppress glucagon secretion which cannot occur in persons with diabetes injecting exogenous insulin. Normally, insulin, also in high concentration, reaches the liver via the portal vein to suppress liver production of glucose. Again, high levels of insulin reaching the liver are not possible by taking exogenous insulin for diabetes. Normally, the suppression of glucagon by insulin also suppresses the production of glucose in the liver. All of these mechanisms are responsible for preventing hyperglycemia both after meals and while fasting. Conversely in healthy subjects if BG falls, for example, during fasting, insulin secretion by the β-cells in inhibited and thus glucagon secretion by the alpha-cells is stimulated both of which increase glucose production primarily by the liver, but also by the kidneys. The liver and kidneys can make glucose from amino acids (from lean tissues), glycerol (from fat), acetone (a ketone made from fat), and from lactate (from muscle, red blood cells, other tissues). Thus, in healthy subjects hypoglycemia is an unusual condition. The third mechanism that corrects hypoglycemia in the unlikely event that the reduction in insulin secretion and increase in glucagon secretion are not sufficient to raise BG, is the sympathetic nervous system which secretes epinephrine (adrenaline), norepinephrine (noradrenaline), and acetylcholine while the adrenal glands secrete epinephrine. Epinephrine and norepinephrine stimulate glucose production by the liver and also result in symptoms of hypoglycemia including increased heart rate, blood pressure, and palpitations. The sympathetic nervous system also secretes acetylcholine which causes sweating and anxiety as well as stimulating glucose production. However, in those with T1DM with complete β-cell destruction the first two mechanisms (decrease in insulin and increase in glucagon) mentioned above are not functioning and recovery from hypoglycemia is dependent on either the person’s own recognition of the problem prior to symptoms via a meter or CGM reading or from the hypoglycemic symptoms caused by the activation of the sympathoadrenal axis.
Hopefully this explains why hypoglycemia almost invariably occurs in persons with T1DM and in those with T2DM who require insulin and/or medications that stimulate insulin secretion and why hypoglycemia is the greatest barrier to achieving near-normal glycemia. In addition to insulin, other medications used for diabetes can also precipitate hypoglycemia. These medications will be reviewed below.
What Should Our Target BG Be To Both Avoid The Long-term Complications Of Diabetes And Avoid Hypoglycemia Which Can Be Fatal?
Achieving a degree of glycemic control that minimizes the risk of developing the long-term complications of diabetes is obviously desirable. These diabetic complications include, but are not limited to, both microvascular complications (neuropathy, retinopathy, diabetic nephropathy) and macrovascular complications (heart disease, stroke, peripheral vascular disease) which can result in blindness, kidney failure, amputations, and cause a reduction in health-span and life-span. However, believe it or not, there is no research that reveals what level of glycemic control is necessary to avoid these complications.
The Diabetes Control and Complications Trial (DCCT) published on September 30, 1993 in the New England Journal of Medicine (ref 1) enrolled 711 persons with T1DM to the intensive insulin arm and 730 to the conventional arm of the study. They had two groups in each arm of the study, one without evidence of diabetic complications (the primary prevention group) and the other with evidence of diabetic complications (the secondary prevention group). The study showed that intensive insulin therapy was able to lower HbA1c from 9% to 7% by increasing doses of insulin, but no changes in diet, which significantly reduced the incidence and progression of diabetic retinopathy (by 76%), neuropathy (by 60%), and nephropathy (of albuminuria by 54%). This was the first large randomized clinical trial to demonstrate this benefit of improved glycemic control. However, this came at a price. The intensive insulin group suffered approximately three times as many episodes of severe hypoglycemia. Severe hypoglycemia is defined as an episode of hypoglycemia that requires the assistance of another person to recover from whether that comes in the form of someone giving the person with T1DM glucose tablets, a glucagon injection, calling 911, or requiring hospitalization (e.g. due to a seizure). Another complication in the DCCT was that at five years, patients receiving intensive insulin therapy gained a mean of 4.6 kg body weight more than patients receiving conventional therapy. Importantly, on secondary analysis, the DCCT could not identify any level of HbA1c that would maximize benefits (i.e. reduce complications) while at the same time minimizing risks (i.e. reduce hypoglycemia).
I have to interject at this point that these limitations of intensive insulin therapy without dietary intervention is where the low carbohydrate ketogenic diet is a potential game changer because glycemic control can be improved significantly with reduced insulin doses (not increased insulin doses as in the DCCT). More on this in my next blog post #53 or see ref 21.
In summary, the DCCT showed that improving HbA1c from 9% to 7% with increased insulin doses significantly reduced the incidence and progression of long-term diabetic complications, but also results in a marked three-fold increase in severe hypoglycemia and a mean 4.6 kg body weight gain.
Unfortunately, we are still left with the question of how low should average BG or HbA1c be to both minimize the risk of long-term diabetic complications and hypoglycemia. I hope it is apparent that the lower the target BG even while on a low carbohydrate ketogenic diet, the more likely hypoglycemia is to occur due to the inherent variability created by using exogenous insulin. A definitive answer will require a long-term clinical trial that utilizes a low carbohydrate ketogenic diet. Until that trial is done, I think each individual will need to determine his/her own glycemic target in consultation with their physician. This glycemic target should be as close to normal as is safely achievable. Now, we need to know what “normal” is and what “safe” is.
What BG Value Is Normal?
This study (ref 2) of 21 healthy non-diabetic subjects wearing continuous glucose monitors (CGM) found that “the mean 24-hour interstitial glucose concentration under everyday life conditions was 89.3 ± 6.2 mg/dl (mean ± SD, where SD = standard deviation), and mean interstitial glucose concentrations at daytime and during the night were 93.0 ± 7.0 and 81.8 ± 6.3 mg/dl, respectively.” Whereas this study (ref 3) of 74 healthy non-diabetic children, adolescents, and adults had a mean interstitial glucose of 98 ± 13.7 (mean ± SD) using a blinded CGM device for 3 to 7 days. A weighted mean of these 95 healthy persons from these two studies reveals that a normal BG is 96 ± 12 mg/dl (mean ± SD) and coefficient of variation is 12.5% (or 12/96 = 12.5%). A mean BG of 96 mg/dl represents, in my opinion, the minimum target BG value to aim for since these healthy subjects would not be expected to develop diabetic complications. Aiming for an average BG less than 96 mg/dl would only increase the risk of hypoglycemia without providing any benefits. If this BG target of 96 mg/dl results in frequent or severe hypoglycemia, then the target average BG should be increased to whatever value is necessary to minimize hypoglycemia. You then might ask, “Does the increased BG variability lead to the development of diabetic complications?” As you may know, BG does not stay in a narrow range in those with diabetes and this BG variability is easily measured by calculating the standard deviation (SD) of your BG readings. Unfortunately, no studies have examined the question: Does BG variability lead to diabetic complications? I think everyone with diabetes is striving to keep their BG variability as low as possible, but actually doing it is difficult. My SD before the ketogenic diet was 54 mg/dl and during the past six years on the ketogenic diet has ranged from 35 to 51 mg/dl over 1 year periods. You can see this is quite high compared to normal at 12 mg/dl.
What Incidence Of Hypoglycemia Is Considered “Safe”?
I do not have the answer to this question, but certainly the lower the better is the best bet. Regrettably, it only takes one severe hypoglycemic episode to die. The lesson here is to not let the zeal of perfection defeat the whole purpose of the pursuit. Personally, I have been striving to keep my BG between 61 and 110 mg/dl more than 70% of time and in addition spending less than 10% of time < 61 mg/dl. Although I am close to that goal every month, it has been difficult for me to actually achieve it. I am working to improve it. For those who do not take insulin for diabetes, it may be difficult to understand how variable the glycemic results of taking insulin can be. For me, this has been the most frustrating part of having T1DM. For example, one day I may wake up with a BG of 90 mg/dl and take 3 units of Humalog with breakfast and get a postprandial BG of 110 mg/dl. The very next day, I may wake up with a BG of 97 mg/dl and take 3 units of Humalog with breakfast and get a postprandial BG of 67 mg/dl having eaten the same breakfast, lunch, dinner, and done very similar exercise type, intensity, and duration. This has happened virtually every day for the past 20 years. Thus, for me anyway, each dose of insulin is a guess and the BG results are unpredictable.
Have You Noticed A Reduction In Symptoms Of Hypoglycemia Since Starting The Ketogenic Diet?
This was the first change that I became aware of after starting the ketogenic diet on Feb. 8, 2012. This started me down a path of investigation to try to understand it better. I personally have had BG values in the 30s mg/dl without symptoms. Please do not confuse this last statement as an indication that asymptomatic hypoglycemia is an acceptable or desirable condition. In fact, this is one of the reasons I decided to write this blog post on hypoglycemia. I am simply stating the fact that since starting my low carbohydrate ketogenic diet, and never before, I have had a significant reduction in the symptoms of hypoglycemia. However, I do not know how much of this reduction in symptoms is due to hypoglycemia unawareness and how much is due to the brain being able to use ketones as a fuel by following a ketogenic diet.
Hypoglycemia Unawareness
Hypoglycemia unawareness simply means that a person with diabetes who has an abnormally low BG is unaware of it because they are not experiencing any sympathetic symptoms.
Additionally, when hypoglycemia is even more severe (< 50–55 mg/dl, < 2.8–3.0 mmol/l), persons with diabetes may not recognize their own neuroglycopenic symptoms including cognitive impairments, behavioral changes, and psychomotor abnormalities, and, at even lower BG levels, seizure and coma. The exact mechanism for hypoglycemia unawareness is still unknown. However, it is known to occur as a result of antecedent episodes of hypoglycemia. The brain somehow adapts to these episodes of hypoglycemia and does not perceive subsequent episodes as an emergency and thus does not activate the sympathetic nervous system and adrenal glands which are in place to correct hypoglycemia by secreting epinephrine (adrenaline), norepinephrine (noradrenaline), and acetylcholine. This adaption is considered by experts in the field to be a double-edged sword (ref 4) because on the positive side the brain does not perceive an emergency requiring secretion of epinephrine which is thought to be the most common cause of harm from hypoglycemia, i.e. lethal cardiac arrhythmias mediated by sympathoadrenal activation (ref 5). Another study (ref 6) in rats found that 3 days of recurrent moderate hypoglycemia resulted in 62–74% less brain cell death after a subsequent episode of severe hyperinsulinemic hypoglycemia (BG 10-15 mg/dl) and were protected from most of the deficits in spatial learning and memory disturbances caused by severe hypoglycemia compared to the control rats. On the negative side of this double-edged sword, the lack of symptoms is thought to lead to more episodes of hypoglycemia any one of which could be severe enough to result in death from neuroglycopenia i.e. not enough glucose for the brain to survive. It is this negative side that needs special attention because we know that depending on the study cited, between 4 and 10% of those with T1DM actually die from hypoglycemia. The majority of these persons die in their sleep which is a time when perception of hypoglycemia is additionally reduced. In addition to antecedent hypoglycemia and sleep, exercise and alcohol ingestion also lead to hypoglycemia unawareness. Although we can’t avoid sleep, and shouldn’t avoid exercise, we can avoid drinking alcohol and work to minimize hypoglycemic episodes. Like many of the followers of this blog, I am a person with T1DM who is highly motivated to achieve BG values as close to normal as is safely possible. However if any of us die or are seriously harmed from hypoglycemia, we have just defeated our own goal of living a normal life-span without diabetic complications. Thus, minimizing or avoiding hypoglycemia is of paramount importance. In fact, this is what I mean by “as is safely possible.” If a person with diabetes is having frequent hypoglycemia, then steps need to be taken to reduce them. I will cover those steps below. Also, be aware that the hypoglycemia unawareness due to recurrent hypoglycemia is completely reversible by avoiding hypoglycemia for 3-4 weeks (ref 7). Resolving hypoglycemia unawareness is an important strategy for preventing future hypoglycemia.
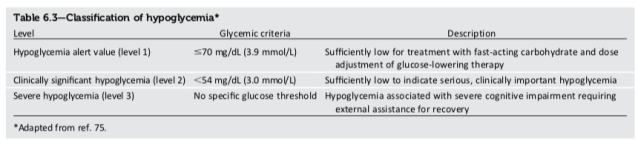
However, at this point you may be asking “given that I follow a ketogenic diet and lack symptoms of hypoglycemia at levels of BG that previously caused symptoms, what value of BG do I use to define hypoglycemia?” Certainly, low BG values should not be accepted as “OK”, even if they occur without symptoms. The 2018 American Diabetes Association (ADA) Standards of Medical Care in Diabetes (ref 8) uses < 70 mg/dl as a threshold to define “hypoglycemia alert value” (see Table 6.3 from ref 8 shown below). This hypoglycemia alert value signals the need to take a fast-acting carbohydrate (glucose tablet(s)) and to adjust the dose of glucose-lowering therapy. I use both of these recommendations on a daily basis since each and every insulin dose I take is adjusted based on my BG results in the previous several days in the context of physical activity. The difficult part of diabetes control is that these previous results can vary significantly from day to day such that each dose is in effect, a guess. Several years ago, I arbitrarily chose < 61 mg/dl (3.4 mmol/l) to define hypoglycemia for myself since I had to choose a value to calculate the frequency of and time during a 24 hour period spent with hypoglycemia to be able to report my results on this blog. However, that does not mean I feel a BG in the 60s mg/dl requires no treatment or no insulin dose adjustment.
As everyone who follows this blog knows, I am not entirely successful at keeping my BG above 60 mg/dl. However, the purpose of starting this blog was to show the actual results of a highly motivated person doing high-intensity resistance exercise and following a ketogenic diet. Despite my lack of complete success, I want everyone to know that I think hypoglycemia is serious and potentially fatal and should be avoided as much as possible.
Can Nutritional Ketosis Provide An Alternate Brain Fuel And Protect Us From Hypoglycemia?
Another topic of research that needs to be done is to measure the degree to which ketones created by the liver by following a ketogenic diet can act as a brain fuel and lead to a reduction in or lack of symptoms of hypoglycemia in those with T1DM. We know from the study done by Drenick et. al. (ref 9) that in non-diabetic obese persons who fasted for 2 months and achieved blood beta-hydroxybutyrate (BHB) levels of 8 mM when given a single dose of insulin to induce severe hypoglycemia suffered no symptoms despite BG values as low as 9 mg/dl (0.5 mmol/l). However, the applicability of this study to those with diabetes following a ketogenic diet is questionable given that the BHB levels are typically in the 0.5 to 3 mM range from nutritional ketosis. Another study (ref 10) in rats found that the cerebral metabolic rate of glucose decreased by 9% for each 1 mmol/l increase in total plasma ketone body concentration in ketotic rats induced by 3 weeks of a ketogenic diet. “The brain’s ability to switch from glucose oxidation towards ketone bodies requires a type of ‘cerebral metabolic adaptation’. This process is not well understood but is thought to be highly associated with the duration and level of ketosis. Ketones are considered to supply up to 70% of the total energy demands once maximal metabolic adaptation occurs.” Another study (ref 11) of 8 healthy male students found that mental alertness was significantly reduced by moderate hypoglycemia (40 mg/dl or 2.2 mmol/l) after an overnight fast while similar hypoglycemia did not reduce mental alertness after a 72 hour fast. BHB levels were not reported in the abstract (I did not purchase the full article). Finally in this study (ref 12), the effect of hyperketonemia on counter-regulatory hormone responses to hypoglycemia was examined in six healthy subjects. The peak adrenaline (epinephrine) response to hypoglycemia fell from 7.97 to 2.6 nmol/l during ketone infusion and the peak noradrenaline, cortisol and growth hormone responses were also significantly lower during ketone infusion at a rate of 3 mg/min/kg body weight which resulted in a 0.58 mmol/l BHB concentration which as you know is achievable with nutritional ketosis. In addition, the study found that the BG required to elicit the counter-regulatory hormone response was lower during the ketone infusion (BG was 2.5 mmol/l (45 mg/dl) during ketone infusion compared to 3.0 (54 mg/dl) mmol/l without ketones).
These data are quite suggestive that keto-adaption at levels of blood ketones achievable with nutritional ketosis may, in fact, be providing the brain with an alternate source of fuel making hypoglycemia less symptomatic and less dangerous. Of course, formal studies of persons with T1DM following a ketogenic diet long-term need to be done to confirm this potential beneficial effect of the ketogenic diet.
How To Handle Glycemic Fluctuations During Illness
All patients with diabetes need to know how to manage BG during illness. The most common illnesses that lead to glycemic fluctuations include infections (most common are pneumonia and urinary tract infections) and gastrointestinal illnesses (some of which are caused by viruses) which often result in nausea, vomiting, reduced food and fluid intake, and diarrhea. These acute illnesses are a stress to the body which responses by secreting cortisol, growth hormone, glucagon, and epinephrine all of which increase BG. Occasionally, patients with diabetes are prescribed corticosteroid medications (e.g. prednisone, methylprednisolone) for various medical conditions. These medications are synthetic versions of the stress hormone, cortisol, and will increase BG often dramatically. Reduced food intake may modify the changes in BG as well. In the setting of illness, the goal is avoid both severe increases in hyperglycemia which can result in diabetic ketoacidosis (DKA) and to avoid hypoglycemia from overly aggressive increases in insulin doses by trying to maintain near-normal BG. The increases in stress hormones often require increased doses of insulin to control hyperglycemia, but accepting mild hyperglycemia during a short illness is much preferred to developing hypoglycemia from overly aggressive increases in insulin doses. Therefore when treating hyperglycemia during illness, it is best to maintain your usual basal insulin dose and use rapid-acting insulin doses to treat hyperglycemia. Rapid-acting insulin typically has a duration of action of 4-5 hours, so dosing them more frequently than every 4-5 hours should be avoided. This 4-5 hour window also varies with the person, so if you have measured BG repeatedly after taking your rapid-acting insulin and know when your BG has stabilized, you could use that time period instead. Taking rapid-acting insulin more frequently that this time period, is called insulin-stacking and can result in hypoglycemia as can an excess dose of rapid-acting insulin. Keeping BG in the 100-200 mg/dl (5.6-11.1 mmol/l) range during an illness should be adequate enough to prevent DKA. Obviously, mildly elevated BG during an illness will have no impact on long-term complications of diabetes. However, overly aggressive insulin doses during an illness can and has caused death from hypoglycemia. On the flip side, a patient with insulin-requiring diabetes who is unable to eat during an illness sometimes severely reduces their insulin doses or just stops insulin altogether. Remember even while fasting (not eating at all), humans need insulin at some basal rate to prevent hyperglycemia. Add to that the stress hormones of illness, and you can see that severely reducing or stopping basal insulin is also a mistake that can result in DKA.
Finally, If you do not feel you are getting control of your BG with the above guidelines, do not hesitate to seek medical attention (calling your physician or going to the emergency room). Developing either severe hypoglycemia or DKA during an illness is life-threatening and both are preventable in the hospital setting.
Additional medications other than insulin are used to improve glycemic control in those with T1DM and T2DM. Some of these medications can increase the incidence of hypoglycemia. These medications are reviewed below.
Sulfonylureas
The first sulfonylurea was discovered in 1942. The first-generation sulfonylureas include chlorpropamide (Diabinese), tolazamide (Tolinase), and tolbutamide (Orinase). The second generation sulfonylureas include gliclazide (Diamicron), glipizide (Glucotrol), glyburide (also known as glibenclamide) (Micronase, Diabeta, Glynase). Glimepiride (Amaryl) is a third generation sulfonylurea. They are the most prescribed medication for T2DM, probably due to their low cost. Sulfonylureas work primarily by stimulating pancreatic β-cells to secrete insulin and by reducing hepatic clearance of insulin as well as by some other mechanisms. Consequently they are effective only when residual pancreatic β-cells are present (typically only in T2DM). This stimulation of insulin secretion, however, is independent of the BG level which can lead to hypoglycemia. In the settings of an excessive dosage of these drugs, hypoglycemia can last many hours and is prolonged in those with renal failure requiring hospitalization. Sulfonylureas over time can exhaust the β-cells leading to progressive β-cell dysfunction and worsening of insulin secretion (secondary failure). Thus, despite better glycemic control in the short term, diabetes could worsen in the long term. Because sulfonylureas increase β-cell insulin secretion in the setting of insulin resistance, the fat cells are encouraged to store fat and these drugs often cause body weight/fat gain. Finally, sulfonylureas are associated with higher rates of cardiac ischemic events and cardiac deaths compared to metformin in multiple different studies. The FDA issued a special warning on this increased risk of cardiac mortality based on the older studies of tolbutamide. The mechanism for this association is currently unknown. Sulfonylureas are effective in most patients and are low cost, but do have the side-effects of hypoglycemia and weight gain. Sulfonylureas are not used for T1DM since there are no β-cells to stimulate.
Meglitinides
Meglitinides work by stimulating the pancreas to release insulin in response to a meal. It closes ATP-dependent potassium channels in functioning β-cells. This blockade of potassium channels depolarizes the beta cells leading to opening of calcium channels and resulting in influx of calcium. Increased intracellular calcium induces insulin secretion. This release of insulin occurs independent of the current BG level and thus can lead to hypoglycemia, although less so than with the sulfonylureas. Drugs in this class include repaglinide (Prandin) and nateglinide (Starlix). Like sulfonylureas, weight gain is another side-effect. Meglitinides are not used for T1DM since there are no β-cells to stimulate.
Pramlintide (Symlin)
Pramlintide is an analog of the peptide hormone amylin. Amylin is co-secreted with insulin from pancreatic β-cells and acts centrally to slow gastric emptying, suppress postprandial glucagon secretion, and decrease food intake. These actions complement those of insulin to regulate BG levels. Amylin is relatively deficient in patients with T2DM, depending on the severity of β-cell secretory failure, and is essentially absent in patients with T1DM. One trial showed pramlintide reduced postprandial BG, with modest reductions in overall glycemia (HbA1c ≈ 0.33%), but another randomized controlled trial was unable to detect any benefit at 6 months in patients with T1DM (ref 14). Pramlintide also induces modest weight loss through control of appetite centers in the brain. Pramlintide can cause hypoglycemia if meal-time insulin doses are not appropriately reduced. The most common side-effect of pramlintide is nausea which tends to decrease with continued use.
Metformin (Glucophage, Glumetza, Fortamet)
Metformin is the first-line medication for T2DM, but may also be useful for those with T1DM. From this paper (ref 13), “In the diabetic state, there is inadequate suppression of postprandial glucagon secretion (hyperglucagonemia) resulting in elevated hepatic glucose production. Importantly, exogenously administered insulin is unable both to restore normal postprandial insulin concentrations in the portal vein and to suppress glucagon secretion [by the alpha-cells] through a paracrine effect. This results in an abnormally high glucagon-to-insulin ratio that favors the release of hepatic glucose. These limits of exogenously administered insulin therapy are well documented in individuals with type 1 or type 2 diabetes and are considered to be important contributors to the postprandial hyperglycemic state characteristic of diabetes.”
So far, researchers have not discovered a way to inhibit this excess glucagon production or to block glucagon receptors. For another helpful review of glucagon in T1DM, check (ref 14). But, this is where metformin may be helpful. Metformin acts on the liver to reduce glucose production by suppressing both gluconeogenesis and glycogenolysis. I speculate that metformin may be especially useful for those with T1DM on a low carbohydrate ketogenic diet because the reduction in dietary carbohydrate reduces insulin requirements. This in turn might stimulate glucagon secretion by the alpha-cells in the pancreas even more than that caused by the diabetic state. This would lead to chronic overproduction of glucose by the liver and contribute to hyperglycemia. However, hyperglycemia does not suppress glucagon production without the accompanying physiologic increase in insulin secretion which does not occur in T1DM. The use of metformin for T1DM while following a ketogenic diet also needs a clinical trial that has yet to be done. Metformin also stimulates muscle uptake of glucose independent of insulin. Although uncommon in those not following a ketogenic diet, metformin might cause hypoglycemia if meal-time insulin doses are not appropriately reduced in those following a ketogenic diet. A meta-analysis (ref 15) of metformin use in those with T1DM not following a ketogenic diet found the following:
“RESULTS: In total, eight randomized controlled trials were included. Metformin was associated with a reduction in daily insulin dosage, body weight, total cholesterol level, low-density lipoprotein level, and high-density lipoprotein level but an increase in risk of gastrointestinal adverse effects compared with placebo treatment in T1DM patients. No significant difference was found between the metformin group and the placebo group in HbA1c level, FPG level, or triglycerides level. No significant difference was found between the metformin group and the placebo group in the risk of severe hypoglycemia or diabetic ketoacidosis.”
Thiazolidinediones
Thiazolidinediones are peroxisome proliferator-activated receptor gamma (PPARg) agonists with multiple actions that lead to improved insulin sensitivity. Drugs in this class include pioglitazone (Actos) and rosiglitazone (Avandia). This class of drugs is used for T2DM where insulin resistance and carbohydrate resistance are the primary defects. Serious side-effects include fluid retention (edema) and hypertension especially when combined with exogenous insulin, upper respiratory tract infections, and headaches. Rosiglitazone was found to increase the risk of myocardial ischemia and heart failure which led Europe and the UK to remove it from the market. Pioglitazone was linked to an increased risk of bladder cancer in one study, but the strength of the data did not warrant removal of the drug from the market. I could find only one small pilot study done in new-onset T1DM which showed pioglitazone did not preserve β-cell function when compared to placebo. (ref 16).
GLP-1 (glucagon-like peptide 1) receptor agonists
GLP-1 is an incretin peptide that is produced and secreted by intestinal enteroendocrine L-cells and certain neurons in the brainstem upon food consumption. The medications in this class have small amino acid substitutions in GLP-1 that bind to and activate the GLP-1 receptor with longer durations of action. They include albiglutide (Tanzeum), dulaglutide (Trulicity), lixisenatide (Lyxumia/Adlyxin), liraglutide (Victoza), semaglutide (Ozempic), and exenatide (Byetta/Bydureon). They are administered by subcutaneous injection. They are most commonly used in T2DM wherein they stimulate insulin secretion in a glucose-dependent fashion, but may be used for T1DM wherein they inhibit glucagon secretion and may inhibit appetite to assist with losing excess body fat. Two trials of GLP-1 receptor agonists for T1DM showed a reduction in insulin requirements without an increase in hypoglycemia (ref 14). GLP-1 receptor agonists have not been studied in those who follow a ketogenic diet.
DPP-4 (dipeptidyl peptidase 4) Inhibitors
DPP-4 is an enzyme that degrades endogenous GLP-1. By inhibiting DPP-4, DPP-4 inhibitors potentiate the activity of GLP-1 or the GLP-1 receptor agonists (see above). This class of diabetes medications include sitagliptin (Januvia), saxagliptin (Onglyza), linagliptin (Tradjenta), vildagliptin (Galvus, Zomelis), saxagliptin/metformin extended release (Janumet XR, Kombiglyze XR), and vildagliptin/metformin (Eucreas). In T2DM, HbA1c decreased by 0.5 – 1.4% depending on the clinical trial and duration of therapy. The most common side-effects include nasopharyngitis, upper respiratory tract infection, and headache. In a meta-analysis of clinical trials regarding treatment with sitagliptin and vildagliptin, there was no increased incidence of hypoglycemic events compared with the control group. Two small clinical trials of sitagliptin and vildagliptin for T1DM showed not only improvement in glucagon levels, but also a modest reduction in HbA1c (ref 14), but a larger longer trial showed no differences (ref 17).
SGLT-2 (sodium/glucose cotransporter) inhibitors
The sodium/glucose cotransporter in the renal tubule of the kidneys functions to reabsorb filtered glucose. Normally, this transporter prevents any glucose from reaching the urine. However in uncontrolled diabetes (T1DM or T2DM), so much glucose is filtered that the transporter cannot keep pace and glucose spills out into the urine (glycosuria). The medications in this class inhibit this transporter and reduces the reabsorption of filtered glucose such that glucose spills out into the urine depending on the BG level. Thus, less glucose appears in the urine when BG is normal which minimizes the potential for hypoglycemia. Because these drugs do not stimulate insulin secretion, exhaustion of β-cells does not occur. They are not effective in the setting of impaired kidney function. This class of medications include ertugliflozin (Steglatro), empagliflozin (Jardiance), canagliflozin (Invokana), and dapagliflozin (Farxiga). They are the newest class of diabetes medications used primarily for T2DM and are not commonly used for T1DM. When used off-label for T1DM, they should be used with caution not only because they could lead to hypoglycemia if insulin doses are not reduced sufficiently, but also because they can cause euglycemic diabetic ketoacidosis (EDKA). The reduction in BG causes both a reduction in insulin secretion (or insulin dosage in T1DM) and an increase in glucagon secretion. Therefore, in both T1DM and T2DM the decrease in the insulin/glucagon ratio, in some instances, can signal the liver to make excessive amounts of ketones leading to diabetic ketoacidosis, but with a normal or only mildly elevated BG i.e. EDKA. Since BG alone is typically measured, the patient may be unaware of the impending problem until they become ill from EDKA. Twenty cases of EDKA were reported to the FDA and 101 cases of EDKA worldwide as of 2015, the majority of whom had a diagnosis of T2DM and were taking exogenous insulin. The FDA also identified potential triggering factors such as intercurrent illness, reduced food and fluid intake, excessively reduced insulin doses, and a history of alcohol intake. Additionally, some cases of insulin-treated T2DM may have been mislabeled and they actually had T1DM. Other side-effects include genital yeast infections, urinary tract infections, dehydration, hypotension, hyperkalemia, and kidney failure. SGLT-2 inhibitors are effective in improving glycemic control, but the above precautions and risks need to be understood.
α-Glucosidase inhibitors
Alpha-Glucosidase inhibitors work by blocking the action of enzymes that normally begin to break down certain carbohydrates in the upper part of the small intestine, thus delaying the absorption of carbohydrates from the intestine. They are used primarily to improve post-prandial hyperglycemia in T2DM. The oral drugs in this class include acarbose (Precose), miglitol (Glyset), and voglibose (Volix). Side-effects include bloating, nausea, diarrhea, and flatulence. Usually, these side effects can be minimized by starting therapy with a small dose and slowly working up to the most effective dose. They do not cause hypoglycemia by themselves, but can slow the absorption of simple carbohydrates other than glucose used to treat hypoglycemia. Thus, pure glucose should be used to treat hypoglycemia. They may be used or either T1DM or T2DM. For those following a very low carbohydrate ketogenic diet, I would not expect these drugs to add any benefit.
Dopamine-2 agonist
Bromocriptine is a sympatholytic D2-dopamine agonist that has been approved for the treatment of type 2 diabetes. Based on animal and human studies, timed bromocriptine administration within 2 hours of awakening is believed to augment low hypothalamic dopamine levels and inhibit excessive sympathetic tone within the central nervous system (CNS), resulting in a reduction in postprandial BG levels due to enhanced suppression of hepatic glucose production. Bromocriptine has not been shown to augment insulin secretion or enhance insulin sensitivity in peripheral tissues (muscle). Addition of bromocriptine to poorly controlled type 2 diabetic patients treated with diet alone, metformin, sulfonylureas, or thiazolidinediones produces a 0.5–0.7% decrement in HbA1c. Bromocriptine also reduces fasting and postprandial plasma free fatty acid (FFA) and triglyceride levels. In a 52 double-blind, placebo-controlled study in type 2 diabetic patients, bromocriptine reduced the composite cardiovascular end point by 40%. The mechanism of the drug’s beneficial effect on cardiovascular disease remains to be determined. Drugs in this class include bromocriptine (Parlodel) and a timed-release bromocriptine mesylate (Cycloset). Side-effects that occurred more commonly in Cycloset versus placebo were nausea (26 vs. 5%), asthenia (15 vs. 8%), constipation (11 vs. 4%), dizziness (11 vs. 6%), and rhinitis (8 vs. 5%). In general, these side effects were mild and transient. Thirteen percent of Cycloset-treated subjects withdrew because of adverse events compared with 3–5% of placebo-treated subjects (P < 0.01). There was no increase in serious adverse events in the Cycloset compared with placebo groups (2.4 vs. 4.3%, respectively). There was no difference in the incidence of hypoglycemia between the Cycloset and placebo-treated groups in any trial. The mechanism(s) via which timed bromocriptine reduces cardiovascular events in type 2 diabetic patients by 40% remains to be defined. Based on the composite cardiovascular outcome, 79 diabetic patients need to be treated for 1 year to avoid one cardiovascular event.
Steps That Should Be Taken To Improve Glycemic Control and Avoid Hypoglycemia
From the discussion reviewed above, the exact target BG that will both maximize benefit (lowest risk of long-term complications) and minimize risk (lowest risk of hypoglycemia) is NOT known. Each person with diabetes needs to discuss with their physician what their target BG value should be to first minimize the number and severity of hypoglycemic episodes. This is a safety-first approach. Once the number of hypoglycemic episodes is minimized, then one can begin to lower the target BG towards a normal average BG reading of 96 mg/dl. If the number/severity of hypoglycemic episodes increases, then the target BG should be increased to a higher value. Thus, the target BG should be titrated down toward 96 mg/dl (but not below) while minimizing the number/severity of hypoglycemic episodes.
When trying to reduce the number and severity of hypoglycemic episodes, one needs to look carefully at several variables. First and most importantly, the basal and/or bolus insulin doses need to be reduced especially when using a low carbohydrate ketogenic diet. Remember that basal insulin (or the basal rate in those who use an insulin pump) covers one’s insulin needs between meals and overnight. The basal insulin dose is determined primarily by the fasting BG results. Fasting hypoglycemia, especially occurring on more than one day, will require a reduction in the basal insulin dose. The meal-time bolus insulin dose is determined primarily by the post-meal BG results. Post-meal hypoglycemia will likely require a reduction in the meal-time insulin dose (assuming similar meal and exercise). The different basal and bolus insulin preparations and methods of administering and adjusting doses are covered in detail in our books mentioned at the beginning of this blog post.
Keeping one’s meals as consistent as possible from day to day will greatly improve the consistency of the BG response to one’s meals. Thus, I for example, attempt to keep the quantity and types of food I eat at each meal consistent from day to day so that the grams of protein, carbs, fat, and fiber remain relatively constant. Similarly, I try to keep my exercise type, duration, intensity, and time of day that I exercise, consistent as well. Exercise has a very significant effect on one’s insulin sensitivity and therefore on the BG response to exogenous insulin.
Exercise and Hypoglycemia
Followers of this blog may know that my interest in the ketogenic diet had its origins when I was trying to improve my glycemic control to complete an ironman distance triathlon in 2012. I started training in swimming, cycling, and running in August 2007 and did my first sprint triathlon on Dec. 8, 2007. I progressively increased the distance each year completing my first olympic distance triathlon Nov. 9, 2008, and my first half ironman distance triathlon on Nov. 8, 2009. As the distance of training increased, I started having hypoglycemia which I did not like so much that I started using sports nutrition products (sugar essentially) preemptively to the point that I was developing hyperglycemia. On Sept. 18, 2009, I had the highest HbA1c ever at 7.9% which I felt was due to this practice of using sports nutrition products. In 2011 I was hoping to complete an ironman distance triathlon, but felt the amount of time required to complete it (around 15 hrs) would require a better approach. I was concerned about both hypoglycemia and hyperglycemia occurring during the event, but hypoglycemia could have resulted in more dire consequences had I been unable to recognize the symptoms. I discovered through listening to two podcasts, IM Talk and Jimmy Moore’s Livin’ La Vida Low Carb, about Dr. Richard K. Bernstein, Dr. Stephen Phinney and Dr. Jeff Volek and the ketogenic diet. I implemented it on Feb. 8, 2012. This was a game changer for me, not only for my life in general, but specifically my need for carbohydrate during exercise and the swings in BG were dramatically reduced. Rather than taking carbohydrate at the beginning of exercise, I measured my BG every hour during exercise and supplemented with carbohydrate on an as needed basis. I stopped having hypoglycemia during exercise which gave me the confidence to complete the ironman distance triathlon on Oct. 20, 2012 in 15.5 hours. This was not a competitive time, but I was just happy to have been able to do it without hypoglycemia and a BG less than 200 mg/dl for the majority of the time. From this experience, I am convinced that the lower insulin doses and fat-adaptation that result from a ketogenic diet allow for improved glycemic control during endurance exercise. The endurance exercise also contributed to improved insulin sensitivity which allowed for additional lowering of insulin doses. One problem I noticed, however, was that the long endurance training did require a day or two of rest to recover which resulted in varying insulin sensitivity from day to day. So I noticed I was adjusting insulin doses more so than what I was doing prior to starting regular exercise. After completing the ironman distance triathlon in 2012, I was so excited about the accomplishment that I continued pushing my training and signed up for another ironman event in April 2013. However, soon before the event all that training finally caught up to me and I was having both knee and foot pains from iliotibial band syndrome and plantar fasciitis. I continued exercising with a lot of swimming and short bike rides. As you might guess I also increased the distance of the swims and did two 5 kilometer swims. These swims were completed on the ketogenic diet so I was not worried so much about hypoglycemia and had none to boot. I never did another triathlon and it took almost 3 years for the plantar fasciitis to finally go away. In Dec. 2014, I started weightlifting mainly to address recurrent low back pain which I been having for years precipitated by lifting things, cycling, and chores that involved bending over. I started with powerlifting: deadlift, squat, bench press. After 3 months, I was again developing overuse injuries, now in my elbows and I had flared up a preexisting injury in my left shoulder doing the bench press. So I switched to olympic weightlifting in March 2015 and over time, my central back pain with radiculopathy consistent with a herniated disc did resolve. It took several years to figure out how much exercise I could do without overtraining and in the process figured out that exercising daily had the most stabilizing effect on my glycemic control. With a few exceptions, I found that olympic weightlifting which I would describe as high-intensity resistance exercise almost invariably increases my BG. The elevation has ranged from mild to rather extreme (an increase of about 120 mg/dl at most). I have accepted these increases as a normal consequence of the type of exercise that I enjoy. It is caused by release of the stress hormones cortisol, epinephrine, growth hormone, and glucagon which serve to release glucose from the liver and fatty acids from fat to supply energy to exercising muscles. This stress hormone effect definitely did not happen during my endurance training exercise since I was doing long-slow training, i.e. no sprinting. Initially, I took small doses of rapid-acting insulin to correct the BG elevations after exercise. Up to that point, I had been avoiding eating lunch for 17 years so as to avoid having to take insulin and thus avoid the chance of hypoglycemia after lunch. So I added a small protein-containing lunch (1/4 lb. hamburger patty and one egg) after my weightlifting session since I was taking insulin most days anyway in the hopes of adding some additional muscle. So far, my muscle mass seems rather stable, but if I can prevent it from declining that will be a victory. If I stop exercising which sometimes happens due to an injury or travel, I develop hyperglycemia using the same insulin doses and thus require increases in insulin doses progressively each day that I do not exercise. After two weeks of no exercise, the insulin doses stabilize at a new higher dose. Several years ago this occurred and I had to increase my total daily insulin dose from 30 IU/day to 42 IU/day. This represents a 40% increase in insulin dosage due to the reduced insulin sensitivity from the cessation of exercise.
All this is a long way of saying that I have had a lot of experience with exercising with T1DM as an endurance athlete on both a high-carb and low-carb ketogenic diet and with high-intensity resistance training on a low-carb ketogenic diet. The lessons I have learned and the reading I have done on the topic include the following:
- Hypoglycemia almost invariably occurred with endurance exercise when not consuming sports nutrition products (sugar) while on a high-carb diet. Longer duration exercise was more likely to result in hypoglycemia.
- Hyperglycemia commonly occurred with endurance exercise when consuming sports nutrition products (sugar) while on a high-carb diet.
- There were days when my BG was well controlled with endurance exercise when consuming sports nutrition products (sugar) while on a high-carb diet, but it seemed to be the minority of the time.
- If I had a CGM, I think I could have done a better job controlling my BG. I have spoken to other T1DM athletes who had a positive experience with a CGM.
- If I had an insulin pump, reducing the basal rate prior to exercise may have allowed for controlled BG during endurance exercise without consuming much sports nutrition products (sugar) while on a high-carb diet, but that is speculation. I have spoken to other T1DM athletes who had a positive experience with this and others for whom it did not work.
- I had much better glycemic control during and after endurance exercise after starting the ketogenic diet. My HbA1c was 5.6% after the ironman distance triathlon. I used very little sports nutrition products (sugar) as a result of the ketogenic diet possibly due to the improved ability to burn fat as a fuel while taking less insulin. I would use glucose tablets instead of sports nutrition products on an as needed basis were I to do it again.
- Post-exercise hypoglycemia can and does occur in many persons with T1DM. For me, this usually occurred after dinner (9 pm) and I learned to reduce my rapid-acting insulin doses at dinner-time to improve it. However, post-exercise hypoglycemia can and does occur in many persons with T1DM during the night while sleeping (nocturnal hypoglycemia). Making sure BG is in your target range before going to sleep and setting an alarm to check your BG or using a CGM with an alarm is a good idea until you can understand your glycemic pattern after exercise. For me, nocturnal hypoglycemia was a problem when taking NPH insulin as my basal insulin and for a period of 3 months (March – May 2008) when I didn’t realize that my BG meter was reading falsely high. On June 6, 2008, my HbA1c was 5.3% (lowest ever on a high-carb diet) with many hypoglycemic episodes. With the combination of glargine (Lantus) insulin and getting the Freestyle Freedom Lite glucose meter, my nocturnal hypoglycemia stopped.
- Intense exercise of any type (resistance, running, cycling, swimming, etc.) can result in hyperglycemia due to the normal stress hormone response to exercise. Normally, insulin is released to control the degree of hyperglycemia during exercise and to normalize the BG after exercise which does not occur in those with diabetes especially T1DM (ref 18).
- In my personal experience, high-intensity resistance exercise on a ketogenic diet usually, but not always, leads to some degree of hyperglycemia (BG > 110 mg/dl). Since this rise in BG occurs in normal athletes, I simply correct it with a dose of rapid-acting insulin after measuring the BG. A larger basal insulin dose might prevent the post-exercise hyperglycemia, but would in turn result in fasting and or nocturnal hypoglycemia which is dangerous. Could I find an exercise that would not affect my BG very much? Probably, but at some point, one should take their enjoyment of physical activity into account. I really like weightlifting.🏋️♀️
Here is a short article on the glycemic response to various types of exercise in those with diabetes (except for the ketogenic part) (ref 19).
Treating Hypoglycemia
One of the most frequent questions I get is whether treating hypoglycemia with glucose tablets/liquid or food for that matter will interfere with ketosis. First, I hope I made it clear above that treating hypoglycemia is essential for one’s own safety. So whether ketosis is affected or not is irrelevant. Hypoglycemia should be treated immediately with anything that is available at the moment. Ideally, every person with diabetes, particularly T1DM, should be carrying glucose tablets/liquid with them at all times. These sources of pure glucose are best for rapidly raising BG and resolving any symptoms of hypoglycemia as quickly as possible. The longer the symptoms last, in addition to being unpleasant, the more potential for more serious complications as well as overcorrection by consuming too much food or sugar. I would like to explain that sugar is sucrose which is a compound composed of one glucose and one fructose molecule. The fructose can be converted to glucose primarily in the liver, but the process is slow compared to the immediate absorption and utilization of glucose. This study (ref 20) found the mean conversion rate from fructose to glucose was 41% ± 10.5 (mean ± SD) in 3–6 hours after ingestion. Thus, sugar is not equivalent to or as good as glucose for correcting hypoglycemia in persons with T1DM (only use it if pure glucose is not available). To treat symptomatic hypoglycemia, take 2-4 glucose tablets (contains 8-16 grams glucose) and wait about 15 mins to see if symptoms are improving. If symptoms are not improving take 2-4 more tablets and seek additional help (e.g. glucagon injection) and/or medical attention. Note that the ADA (ref 8) recommends using 15-20 grams of glucose initially. However, I found this usually results in hyperglycemia. Thus, each person needs to determine their own glucose dose because their body weight and insulin sensitivity will affect the dose of glucose needed to correct hypoglycemia. Taking excessive amounts of food, sugar, or even glucose tablets can result in hyperglycemia which will need to be treated with one or more additional insulin doses. This has and likely will happen to each and every person with T1DM, but these episodes can be minimized by following the above guidelines. Finally, glucose tablets are also best for correcting mild asymptomatic hypoglycemia. I typically use 1/2 or 1 glucose tablet (2-4 grams of glucose). The tablets are scored and easy to break in half. Liquid glucose can be used as well. Finally, recheck BG with your meter to confirm that the hypoglycemia has been corrected especially before driving a car or going to sleep.
Now for the question about what happens to ketosis in those following a ketogenic diet after treating hypoglycemia with glucose tablets. Taking just a few glucose tablets is not likely to affect ketosis at all. In fact because hypoglycemia in those with T1DM is a hyperinsulinemic hypoglycemia, the excess insulin itself is more likely to have already inhibited ketosis if it has been affected at all. You see, insulin inhibits the rate-limiting enzyme, HMG CoA synthase, required to make ketones. Treating hypoglycemia with glucose tablets will effectively use up the excess insulin and may restore ketone synthesis. Thus treating hyperinsulinemic hypoglycemia with glucose tablets will either improve nutritional ketosis or not affect it at all.
Stay tuned for the next blog post #53 on a study (ref 21) just published in the journal Pediatrics where I was one of the participants in the study.
References
- The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. https://www.nejm.org/doi/pdf/10.1056/NEJM199309303291401
- Continuous Glucose Profiles in Healthy Subjects under Everyday Life Conditions and after Different Meals. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2769652/pdf/dst-01-0695.pdf
- Variation of Interstitial Glucose Measurements Assessed by Continuous Glucose Monitors in Healthy, Nondiabetic Individuals. http://care.diabetesjournals.org/content/diacare/33/6/1297.full.pdf
- Hypoglycemia-Associated Autonomic Failure in Diabetes: Maladaptive, Adaptive, or Both? http://diabetes.diabetesjournals.org/content/64/7/2322
- Severe Hypoglycemia–Induced Lethal Cardiac Arrhythmias Are Mediated by Sympathoadrenal Activation. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3781452/
- Recurrent Moderate Hypoglycemia Ameliorates Brain Damage and Cognitive Dysfunction Induced by Severe Hypoglycemia. https://www.ncbi.nlm.nih.gov/pubmed/20086229
- Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM here https://www.ncbi.nlm.nih.gov/pubmed/7958494
- Standards of Medical Care in Diabetes – 2018. http://care.diabetesjournals.org/content/diacare/suppl/2017/12/08/41.Supplement_1.DC1/DC_41_S1_Combined.pdf
- Resistance to Symptomatic Insulin Reactions after Fasting. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC332976/
- Ketosis proportionately spares glucose utilization in brain. https://www.ncbi.nlm.nih.gov/pubmed/23736643
- Mental alertness in response to hypoglycaemia in normal man: the effect of 12 hours and 72 hours of fasting. https://www.ncbi.nlm.nih.gov/pubmed/3315761
- Ketone infusion lowers hormonal responses to hypoglycaemia: evidence for acute cerebral utilization of a non-glucose fuel. https://www.ncbi.nlm.nih.gov/pubmed/1653662
- Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. http://spectrum.diabetesjournals.org/content/17/3/183
- Alpha cell function in type 1 diabetes. http://www.bjd-abcd.com/index.php/bjd/article/view/12/37
- Efficacy and safety of metformin for patients with type 1 diabetes mellitus: a meta-analysis. https://www.ncbi.nlm.nih.gov/pubmed/25369141
- Effect of Pioglitazone on the Course of New-Onset Type 1 Diabetes Mellitus. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3890222/
- Effect of Sitagliptin on Post-Prandial Glucagon and GLP-1 Levels in Patients With Type 1 Diabetes: Investigator-Initiated, Double-Blind, Randomized, Placebo-Controlled Trial. http://journals.aace.com/doi/abs/10.4158/EP12100.OR
- Differences in the metabolic and hormonal response to exercise between racing cyclists and untrained individuals. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1308956/
- Blood Glucose Responses to Type, Intensity, Duration, and Timing of Exercise. https://digitalcommons.odu.edu/cgi/viewcontent.cgi?article=1048&context=hms_fac_pubs
- Fructose metabolism in humans – what isotopic tracer studies tell us. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3533803/pdf/1743-7075-9-89.pdf
- Management of Type 1 Diabetes With a Very Low-Carbohydrate Diet. http://pediatrics.aappublications.org/content/early/2018/05/03/peds.2017-3349

Congratulations, Keith Runyan, and thanks for the post. Very useful.
LikeLike
Thanks, Eric. I hope it will help others.
LikeLike
It is the third time I read the post. I mean, it is excellent. Really.
Thank you again, Keith.
LikeLike
Outstanding!
Sent from my iPhone
>
LikeLike
Thanks, Caroline. Much appreciated.
LikeLike
Thank you for that comprehensive information! And Happy birthday!
LikeLike
Thank You, Svet.
LikeLiked by 1 person
Keith – I have some questions about hypoglycemia. I tried an experiment where I more-or-less fasted for 5 days. (I was trying to emulate the fasting mimicking diet in the hopes that I’d regenerate beta cells). Anyway, during the “fast,” I reduced my Lantus dosage to 1U per day – and no bolus. To my surprise, I spent most of the days with blood sugars in the 50mg/dL – 60mg/dl range. I determined that this was safe because I observed that other non-diabetics were fasting and having similar blood sugars. I even had a couple readings in the 40s. My first question is: how do you think this is possible? After a few cycles, I tested my c-peptide STIMULATED, and had a result of 0.16 ng/mL – so I’m pretty certain that insulin was not contributing much to my very low blood sugars! After the fast, dosages shot back up to normal quickly. My second question is: Do you think it is possible to have hypoglycemic ketosis? I can only easily measure urine ketones (which is only part of the picture, I know), and found them to always be “small” during the fasting. Of course, I’m fairly well adapted to a very low carb diet (at the beginning, my urine ketones would be large, but now-a-days they are always small). What do you think was going on during this time, and is it dangerous?
LikeLike
Hello again Brian. I am guessing that you are referring to this paper. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5357144/pdf/nihms849263.pdf
by Valter Longo, et.al. where they showed that repeated cycles of the fasting-mimicking diet resulted in partial beta cell regeneration in mice treated with streptozotocin (a toxin that destroys beta cells).
Regarding hypoglycemia after fasting in non-diabetics, I found this paper
https://www.ncbi.nlm.nih.gov/pubmed/3661473
that did show a reduction in plasma glucose from 92 to 68 mg/dl after 3 days of fasting, so I does seem reasonable to think that others could have a more dramatic response to fasting. That paper also showed the normal to fasting: a reduction in insulin and an increase in both glucagon and epinephrine.
The situation is different in T1DM because you are having to regulate your blood glucose with exogenous insulin. Because Lantus has a half-life of 12 hours and you were taking Lantus presumably in progressively smaller doses, the Lantus was contributing to the hypoglycemia. Of course, it would be difficult to know in advance what your insulin requirements would be, so fortunately nothing bad happened. I agree that endogenous insulin was not contributing to the hypoglycemia.
Yes I think it is possible to have hypoglycemic ketosis in the setting of T1DM, fasting, and taking exogenous insulin. I think the ketosis is a result of fasting, called starvation ketosis or starvation ketoacidosis depending on how severe the ketosis is. The hypoglycemia is a result of more insulin than you needed at the time. Is that dangerous? Yes, I think hypoglycemia is always potentially dangerous. I think that because we have T1DM and have hypoglycemia so often that we become complacent and view it as not so bad. I have been guilty of that myself. Now more so than in the past, I am much more concerned about hypoglycemia than I am hyperglycemia simply because the acute consequences are so dramatically different. It only takes one severe episode of hypoglycemia to die, whereas, multiple episodes of transient hyperglycemia may not result in any damage.
Although I understand why you did your experiment, in general IMO, fasting with T1DM will generate more problems than it solves and should be avoided.
LikeLiked by 1 person
Keith – thank you for your thoughtful reply. Sorry for not citing the paper. Yes, you found it. I understand that there were two big issues why what is in the paper might not apply to T1d: #1-These were mice, #2-The mice were treated with SZT which is not a great model for the continuing immune attack present in T1d. In case you’re curious, I did 2.5 cycles, and witnessed no improvement in C-peptide. FWIW, I found a group online where others had completed more cycles and experienced no improvement either. I’m not ready to kiss this idea goodbye, but it’s definitely low priority at this time until more research becomes available. The part that intrigued me was that I could effectively go without insulin if I didn’t eat. If I don’t exercise much, my TDD balloons to ~35U per day, 24U of which is Lantus (bolus portion is small at ~11U due to low carb diet). My progression during the “fast” (of about 400 calories per day) was 4U, 2U, 1U, 1U of Lantus, each day respectively. Do you still believe that on the 4th day, I had much insulin active? A big question I’ve had is: what is the primary function of insulin – does it primarily stop the liver from making/releasing glucose… or does it primarily increase skeletal muscle uptake? I did an experiment where I ate some fruit and slightly under-dosed 5U. I waited and hit ~210mg/dl. The 5U couldn’t bring me down. I ran (easy) for just five minutes. In only 5 minutes, I dropped to ~130mg/dL. Then I sat around for ~30 minutes, at my BG stayed put at ~130mg/dL, creeping up to ~140mg/dL. After the wait, I ran another 5 minutes, and in that time, it dropped another 50mg/dL. So, what happened? Were the muscles preferentially using glucose because there was insulin available, or was the outflow of glucose from the liver being blocked during that time? Fast forward to day 3 of the fasting experiment: Just 1U of Lantus on board (and maybe some residual from the 2U the day before…), and I go for a bike ride. I feel fine, and BG is pretty steady 60-70mg/dL with no food. What is happening? Are the muscles running exclusively on fat, or can the glucose somehow enter the cells with this tiny amount of insulin? Can a glycogen-devoid liver manufacture glucose at a rate sufficient to fuel exercise? How is the BG maintaining a relatively steady state on its own? I hope this isn’t pounding you with too many questions, but I feel like there is still some mystery in understanding how insulin works. My concerns (and questions) about hypoglycemic ketosis cropped up when I came to the realization that if I’m exercising with hardly any available carbohydrate (such as day 3 of the “fast”), that could potentially promote profound ketosis (however, this was not observed in urine for some reason)… Usually we use a high blood sugar in combination with ketones to diagnose ketoacidosis, but my realization was that it may be possible to have ketoacidosis in the absence of high blood sugars under these circumstances, which you have confirmed. But, if I’m taking zero exogenous insulin, fasting and maintaining normal (or low) blood sugars somehow, I do wonder how this is any different from a non-diabetic in the fasted state. Thanks again for your thoughts!
LikeLike
That’s a lot of questions, all good. I’m not sure I have all the answers, but I’ll try.
Do you still believe that on the 4th day, I had much insulin active?
You can tell the amount of active insulin relative to your current insulin sensitivity by your current blood glucose (BG). If your BG is normal, your insulin is just right. If your BG is low, you have too much insulin. If your BG is high, you have too little insulin.
What is the primary function of insulin – does it primarily stop the liver from making/releasing glucose… or does it primarily increase skeletal muscle uptake?
Insulin has many functions and most tissues and organs in the body have insulin receptors. Insulin does suppress glycogenolysis and gluconeogenesis in the liver and it does stimulate skeletal muscle glucose uptake. Also note that skeletal muscle can take up glucose without insulin being present and the higher the BG the more glucose the muscle can take up. That might explain the drop in BG after running when your BG was high.
Are the muscles running exclusively on fat, or can the glucose somehow enter the cells with this tiny amount of insulin?
I would guess your muscles are using both fat and glucose, but with a BG of 60-70 mg/dl there was not a large glucose gradient to drive glucose into the muscle cells and not much insulin around to help either. The lack of insulin in your system (and therefore higher levels of glucagon) was also telling your liver to make glucose. I doubt the liver ever runs out of glycogen even on a low carb diet. The liver’s default mode is to make glucose, with insulin being the only hormone that can reduce it and numerous other hormones that can increase liver glucose production: glucagon, cortisol, epinephrine, norepinephrine, and growth hormone.
Exercise alone promotes ketone production. When combined with fasting and having type 1 diabetes (T1DM), that is a set-up for ketosis and ketoacidosis. I spoke with a T1DM physician who did a 14 day fast and then measured serum bicarbonate (often reported as CO2 on a metabolic panel) at the end of the fast. It had dropped from a normal of 24 meq/L to 14 meq/L indicative of metabolic acidosis and her blood beta-hydroxybutyrate was about 5-6 mM. Fortunately, she felt well, but that was clearly ketoacidosis. Her glucose was normal and she had appropriately decreased her insulin dose dramatically just as you did. Thus, my diagnosis would be starvation ketoacidosis. This is one of the reasons prolonged fasts are not a good idea, especially in those with T1DM. As I said before, the negative side-effects of fasting with T1DM far outnumber any possible benefits. So to your question, yes, non-diabetics can get starvation ketoacidosis as well.
Also, if you do a Google search or PubMed search with “euglycemic diabetic ketoacidosis” you will read about some cases where the person was either sick and couldn’t eat or was fasting and ended up with ketoacidosis and a near-normal glucose.
I hope that helps.
LikeLike