This is a monthly update on my glycemic management of type 1 diabetes (T1DM) using Humalog and Lantus insulin injections with resistance exercise and a ketogenic whole-food diet as described in my book, The Ketogenic Diet for Type 1 Diabetes also available on Amazon in print. My other book, Conquer Type 2 Diabetes with a Ketogenic Diet, is also available on Amazon in print.
Although glycemic management in T1DM will always be challenging, the low carbohydrate ketogenic whole-food diet definitely improves it and just as importantly reduces insulin requirements and frequency of symptomatic hypoglycemia. Many of the diseases (cardiovascular disease, cancer, Alzheimer’s, and many more) associated with T2DM and “double diabetes” as part of T1DM are due to insulin resistance and hyperinsulinemia. The low carbohydrate ketogenic whole-food diet directly improves both insulin resistance and endogenous hyperinsulinemia in T2DM and exogenous insulin requirements in T1DM (i.e. reduced insulin doses).
Last month I detailed my treatment plan for my presumed left rotator cuff injury. Although it seems to be slow to recover, it has improved significantly throughout the months of October, November, and December. I started back doing snatch overhead squats on October 27th and snatch and clean and jerks on November 26th, but stopped doing front squats, snatch, and clean and jerks on December 20th. So far no shoulder pain during exercise, but some soreness with certain arm movements in daily life. I interpret this as much improvement, but not as yet complete recovery. In December, I continued once daily weightlifting workouts but had to decrease from three exercises per day to two per day due to overtraining (again). I have previously discussed the change in blood glucose (BG) with exercise. I still experience a significant rise in BG during weightlifting which I felt was due to stress hormone release from intense exercise (see the black circles i.e. 2 pm BG in the graphs below). However, I am thinking that in addition to that the BG response to exercise is also related to the adequacy or inadequacy of the basal insulin dose. What I have noticed is that when the fasting BG is normal (BG 61-110 mg/dl), the BG rises during weightlifting. But when the fasting BG is low (BG < 61 mg/dl), the BG does not rise during weightlifting and when the fasting BG is high (BG > 110 mg/dl), the BG rises even more dramatically during weightlifting. Of course, there is a lot of variability and these are general observations, not rules or predictable responses. For me, there does not seem to be one basal insulin dose that will result in both a normal fasting BG and a normal post-exercise BG. Several years ago I tried splitting up the basal insulin dose to a morning and dinner-time dose to address this issue with no improvement. For safety reasons, I think it is best to adjust the basal insulin dose (Lantus) to achieve a normal fasting BG and accept and treat an elevated post-exercise BG with a correction dose of rapid-acting insulin (Humalog). I have taken a small correction dose of rapid-acting insulin (Humalog) (about 1-2 IU) to correct a high post-breakfast BG prior to exercise with success most of the time. In December I noticed my post-exercise BG was increasing more dramatically and I thought since I am regularly having to take a dose of rapid-acting insulin and would like to increase my muscle mass further, I added a post-exercise meal consisting of 1/2 lb. ground beef and 18 olives and a larger rapid-acting insulin dose which I started on December 5th.
Glycemic Management Results for December 2017
My December glycemic results were similar to previous time periods with more hyperglycemia than I would have liked. I did have much less hypoglycemia than usual and no symptoms of hypoglycemia. However, I spent only 58% of time with a BG between 61 and 110 mg/dl (my goal is >70%). My insulin doses had to be increased steadily throughout the month to levels I haven’t seen since before starting my ketogenic diet in 2012. Perhaps that was in part related to adding an additional meal per day, but also more of that oscillatory pattern that has been going on for years now. I had to increase the total daily insulin dose from 33 to 65.5 units/day.
Below are my mean BG values, mean insulin doses, and BG frequency distribution for December 2017 compared to previous time periods. I have changed two columns to indicate the AUC mean BG and predicted HbA1c. AUC mean BG is the mean BG by calculating the area under the curve (AUC) of BG versus time. The predicted HbA1c uses the formula: AUC mean BG plus 88.55 divided 33.298. This formula is the least squares fit using my own personal mean BG versus measured HbA1c over many years. My particular HbA1c values are higher than many other individuals with the same mean BG. This is referred to as being a “high glycator.”

As discussed previously, exogenous insulin cannot mimic normal insulin secretion, so persons with T1DM should not expect to have truly normal BG values at all times. They just need to be low enough to prevent long-term complications and not so low as to cause unpleasant hypoglycemic symptoms or less common but dangerous consequences including brain damage, seizure, injury, coma, or death. I have set my target BG range at 61-110 mg/dl because values in this range are not likely to lead to harm or complications of T1DM. Your target BG range should be determined with your physician because one size does not fit all. Normal BG is 96 ± 12 mg/dl (mean ± standard deviation (SD)) and coefficient of variation is 13% which is the weighted mean from these two studies (here and here) of continuous glucose monitoring in healthy subjects. The standard deviation and coefficient of variation are measures of BG variability which I believe are important in T1DM. However, be advised that clinical outcomes in T1DM (i.e. microvascular and macrovascular complications) have only been documented to correlate with measures of mean BG, particularly HbA1c. This does not mean that BG variability is not important, but it just has not been documented to correlate with outcomes and complications of T1DM. Achieving a normal standard deviation or coefficient of variation in T1DM would be difficult, if not impossible, with current exogenous insulin therapy (injected or pumped). Monitoring the standard deviation and/or coefficient of variation and finding ways to improve them to the best of one’s ability is desirable in my opinion. Following a low carbohydrate ketogenic diet is one such method of reducing BG variability, mean BG, insulin doses, and hypoglycemia. A ketogenic diet may also provide an alternate/additional brain fuel in the form of ketones to protect the brain when BG does go low. The alternative energy that ketones supply to the brain may prevent or blunt the sympathoadrenal response to hypoglycemia which in turn reduces or eliminates the symptoms of and harm from hypoglycemia. This hypothesis needs to be tested before it can be stated as fact. Having BG close to normal most of the time (some of which are hypoglycemic) also minimizes the symptoms of mild hypoglycemia and potentially the harm from hypoglycemia as well due to lack of activation of the sympathetic nervous system and adrenal gland responses to hypoglycemia i.e. sympathoadrenal-induced fatal cardiac arrhythmia, see here.
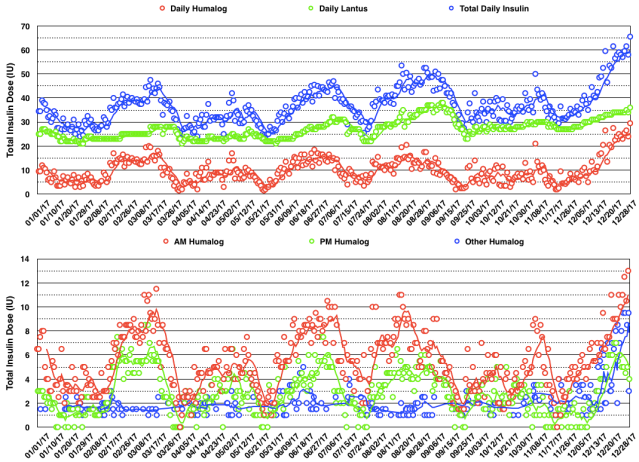
Below are my BG readings along with the Humalog (rapid-acting insulin) doses in December 2017. I adjust both the morning and evening meal-time doses based on the pre-meal BG reading and take extra correction Humalog doses (green circles) for high BG readings between meals and my new post-exercise meal. I continued my previous pattern of high BG readings after weightlifting. This is primarily controlled by the basal insulin (Lantus) dose taken at dinnertime but that dose is determined by the fasting BG reading and thus cannot be adjusted to optimize all times of day. In those with T1DM the basal insulin dose may be enough to compensate for the increase in BG with intense exercise, but may also require a rapid-acting insulin dose to lower a high post-exercise BG.

The table below shows the BG variability results for current and previous time periods. The percentiles (10th, 25th, 75th, 90th) on the right show the spread of the BG readings about the median. The interquartile range, the difference between the 75th and 25th percentiles, is a measure of BG variability. In the middle of the table are the %Time in three BG ranges: %Time BG < 61 mg/dl (hypo) and the mean BG during that time, then %Time BG 61-110 mg/dl (target) and the mean BG during that time, and %Time BG > 110 mg/dl (hyper) and the mean BG during that time. Both the %Time with hypoglycemia and hyperglycemia are probably overestimates because they do not account for the corrections with glucose tablets (for hypoglycemia) or rapid-acting insulin (Humalog) (for hyperglycemia). Measuring my BG more frequently or using a CGM would result in a more accurate estimate.

The daily insulin dose totals and BG readings for December are shown in the graphs below. You can see a steady increase in total daily insulin dose during the month to address hyperglycemia.

The daily insulin dose totals and 7-day moving average for 2017 are shown in the graph below. You can see an oscillatory pattern with a period of about 8 weeks except for the past two months during which the total daily insulin dose steadily increased. Again this may in part be related to the additional meal per day and an increase in body weight of 4.4 lb.
The graph below shows all my BG measurements in 2017. I realize there are so many points that it is difficult to make much sense of it, however, I include it for completeness sake.

In January, I will continue olympic weightlifting every day with two exercises per day. I will also restart metformin and see if I can tolerate it starting with a small dose (250 mg) and slowly increasing it over time as I have always told my patients.
My Thoughts About Management of Type 1 Diabetes With A Ketogenic Diet
My goal of glycemic management in T1DM with a ketogenic diet is to keep BG as close to normal i.e. 96 ± 12 mg/dl (mean ± SD) as is safely possible (i.e. avoiding hypoglycemia) to avoid diabetic complications, a reduction in lifespan, and unpleasant symptoms of as well as injury and death from hypoglycemia. For me, a well-formulated whole-food nutrient-dense ketogenic diet, daily exercise, frequent BG measurements, and lower insulin-analog doses (Humalog/Lantus) have improved my glycemic control, hypoglycemic reactions, and quality of life. My basic diet philosophy is to avoid processed foods especially those containing refined carbohydrates and sugar, while enjoying whole foods (one ingredient) as close to their original state as possible. I think most know that processed foods are made in factories with many ingredients for the purpose of prolonging their shelf life and increasing their addictiveness. Therefore, they use both salt and sugar which serve both those purposes. They also use vegetable oils (seed oils) because some fat is necessary in the manufacturing process and for mouth feel. Another common feature of the ingredients of processed foods is that they are subsidized by the U.S. government and therefore the market price is artificially reduced. Then the food industry “adds value” to these ingredients by their formulation and convenience and then markets them heavily resulting in a very profitable commodity to the food industry. Dr. Robert Lustig has said that sugar is added to 80% of processed foods. Many also have discovered that the majority of these foods can be avoided by shopping on the perimeter of the grocery store in the produce, meat, and dairy sections while avoiding the bakery, deli, and most of the center isles (of course there are some exceptions). I think just knowing the guidelines in this paragraph would be a good start for those wanting to improve their diet in 2018.
My current version of ketogenic diet is as follows:
What I Cook & Eat
- Beef, grass-fed, including meat (85% lean), heart, liver, and kidney (liverwurst)
- Fish, mainly wild Alaskan salmon
- Canadian bacon (uncured pork loin)
- Lamb occasionally
- Chicken & Turkey occasionally
- Chicken Eggs
- Non-starchy vegetables (about 5% carbohydrate content by weight) including Cabbage (Red, Green, Napa), Kale, Collard Greens, Leeks, Onions, Home-made Sauerkraut from Red Cabbage, Bok-Choy, Broccoli, Cauliflower, Yellow Squash, Zucchini, Cucumber, Lettuce (Iceberg & Romaine), and some others.
- Fruit – Avocado, Tomatoes, Olives, lemon juice on fish and salads
- Root Vegetable: Raw Carrots
- Nuts & Seeds – Pepitas, Macadamia, Brazil, Pecan, Walnut, Pistachio, Cashew.
- MCT oil – a few tablespoons on salads or cooked vegetables
- Note: I developed an intolerance to milk prior to my diagnosis of T1D. I did try heavy whipping cream after starting my KLCHF diet, but am also intolerant of it. I do tolerate butter, but wanted to decrease my fat intake, so eliminated all dairy including cheese and yogurt.
What I Drink
Water (filtered by reverse osmosis), Unsweetened Tea & Coffee
What I Don’t Eat
- Grains – Wheat, Corn, Rice, Oats (there are many more) or anything made from them, which is too numerous to list here. Gluten is a protein present in a number of grains (all varieties of wheat including spelt, kamut, and triticale as well as barley and rye.) which can cause a number of medical problems for a significant portion of the population with gluten sensitivity or celiac disease. In my case, I avoid them due to their carbohydrate content.
- Starchy and most root vegetables – potatoes, sweet potatoes, yams
- Legumes – peas, beans, lentils, peanuts, soybeans
- High sugar fruits – includes most fruits except berries, see above.
- Sugar and the fifty other names used to disguise sugar.
- Vegetable Oils – Canola, Corn, Soybean, Peanut, Sunflower, Safflower, Cottonseed, Grape seed, Margarine & Butter substitutes, Shortening.
- All Processed Foods.
- I avoid restaurants except when traveling, and then order fish or steak with plain steamed non-starchy vegetables (no gravy or sauces that typically contain sugar, cornstarch, or flour) or salad.
- Refined, but healthy, fats – Although there is nothing bad about including butter, coconut & olive oil in a ketogenic diet, I have eliminated refined fats except a small amount of MCT oil from my diet to improve my body composition.
What I Don’t Drink
- Colas (both sweetened and artificially sweetened).
- Fruit Juice except small amounts of lemon juice.
- Alcohol (can cause hyperglycemia or hypoglycemia in persons with diabetes).
- No artificial sweeteners, don’t want or like them.
My exercise regimen negatively affects glycemic control, but I enjoy exercising and feel it has health and lifespan-extending benefits which may compensate for the temporary increase in BG during/after exercise. Hopefully my BG values and variability as well as the relatively lower insulin doses that result from my ketogenic diet and exercise are close enough to optimal to avoid any reduction in lifespan, diabetic complications, and harm from hypoglycemia, but only time will tell.

Do you know much about Monogenic forms of diabetes, such as GCK-MODY…especially as related to a ketogenic diet?
LikeLike
Although I have not read about and do not know anyone who has used a ketogenic diet to treat MODY2, the condition is like other forms of diabetes in that it is a state of carbohydrate intolerance due to mild insulin deficiency. Therefore, the condition should be improved with a well-formulated low carbohydrate ketogenic diet.
LikeLike
I encouraged a T1 to switch to Tresiba and the results have surpassed what I expected. I recommended a morning and BEDTIME dose (not dinnertime). This eliminated a pretty severe dawn effect completely. The dose dropped. Previous dose of Levemir was 18u @ dinner and 23u am. Tresiba dose is now 3u a bedtime and 6u am. I am pleased with the 75% reduction in basal insulin. He is an athlete like yourself and weighs 149lbs.
I am not sure why you are reducing fat unless you are trying to lose weight. I have found that if reducing calories pretty low does not work that I have to recommend a reduction in protein but I don’t recommend going below 54 g/day.
LikeLike
Jamie, on a ketogenic diet, dietary protein intake should be in a range of 1-1.6 grams/kg body weight/day. If one has excess body fat, the calculation could be based on desired body weight rather than actual body weight. Whereas, carbohydrates are generally less than 50 grams/day, that could be net carbs which subtracts fiber grams that come from vegetables, nuts, and seeds. That leaves dietary fat which is adjusted to achieve/maintain a desired body composition. For myself I found that by eliminating butter, coconut oil, and olive oil, my body composition improved without having to count calories or grams of anything on any regular basis. In order to lose “weight” any macronutrient could be reduced, however reducing protein primarily would tend to result in loss of lean muscle mass which is not desirable. So IMO the best strategy to lose excess body fat on a ketogenic is to keep protein adequate, carbs very low, and reduce dietary fat just enough to achieve one’s desired body composition.
I have not used Tresiba, but I know it works well for many others.
LikeLike
I reversed neuropathy immediately and early kidney disease in 6 months in a T1 by reducing carbs to <30 g/day. I was asked to give a talk about the case for scientists and providers. I found 1 case report by Jorgensen to support the improvement in renal function in a T2 (see reference below). I believe you are a nephrologist. Can you tell me whether it is standard for diabetics with CKD to be placed on low carb diet and what evidence is used to support this? I have met 2 people with diabetic CKD who were placed on a low carb diet (one stage 3a), so there must be some evidence to support this.
"A low-carbohydrate diet may prevent end-stage renal failure in type 2 diabetes. A case report"
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1523335/
LikeLike
Lisa, unfortunately it is not standard practice to recommend a low carb diet for those with diabetic nephropathy. It should be recommended IMO. I can’t recall any published studies showing either stabilization of renal function (not reversal) as in the case report you sited, or reversal of diabetic nephropathy. Dr. Richard Bernstein has spoken and written about his complete reversal of proteinuria with a low carb diet and he apparently had reduced renal function which normalized although I do not know what his serum creatinine was before starting his low carb diet. I’m glad you’re spreading the word. Eventually it will catch on.
LikeLike